Vaccination remains a key strategy to mitigate the impact of adverse health outcomes on patients and hospital operations during the 2024-2025 respiratory season. The MHA urges all birthing hospitals to become Vaccines for Children (VFC) specialty providers to offer Beyfortus and expand access to eligible patients ahead of the upcoming respiratory season.
Beyfortus was approved by the FDA in July 2023 for preventing RSV lower respiratory tract disease in infants. Hospitals can acquire Beyfortus through direct/private purchase or through the VFC program. However, doses obtained through the VFC program can only be administered to patients who are eligible for the VFC program.
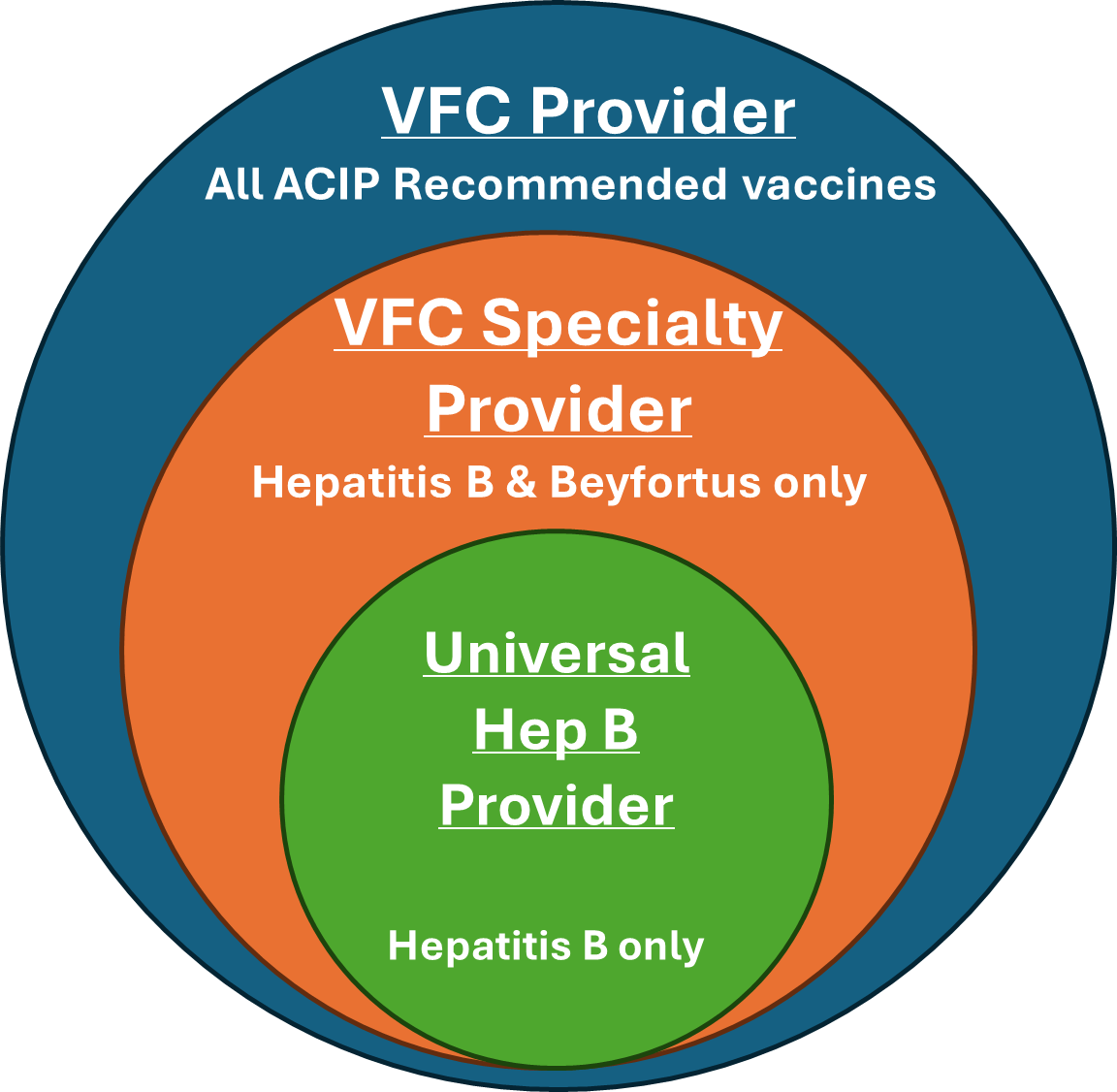
Birthing hospitals must become a VFC enrolled provider to order Beyfortus through the program. While there are three different types of VFC participation, only two allow for administration of Beyfortus:
- VFC Provider (providing all ACIP recommended vaccines)
- VFC Specialty Provider (providing Hepatitis B & Beyfortus only)
The Michigan Department of Health and Human Services (MDHHS) and the Centers for Disease Control are encouraging all birthing hospitals to become a VFC Specialty Provider to protect Michigan infants against Hepatitis B and RSV. The process to become a VFC Specialty Provider differs slightly, depending on if a hospital is participating with the VFC as a Universal Hepatitis B Provider or not at all.
The steps in the graphic below outline how birthing hospitals can become a VFC Specialty Provider:
1 – MDHHS has created a Beyfortus Eligibility Tool to help birthing facilities with this process. Use of the tool is optional, however if it is used, it should be noted in the Eligibility Screening Plan in step two.
2 – Facilities should complete the Eligibility Screening Plan and submit it to the local health department.
Members with questions may contact Kelsey Ostergren at the MHA. Questions related to VFC program enrollment can be directed to the MDHHS Division of Immunizations.